Sertifikasi Manufaktur Plastik Esensial: Kesesuaian ISO, FDA & Kelayakan Makanan
Are you worried that a single safety audit failure could shut down your entire bottle production line? Navigating the complex world of food safety rules is scary, but getting it wrong is not an option.
To ensure food-grade safety, you need strict adherence to FDA 21 CFR regulations for material chemistry and ISO 22000 for process hygiene. For Chinese machinery, verify ISO certificates through the official CNCA database to avoid fraud. These standards prevent chemical migration and ensure your packaging is legal for the US market.
Let’s be honest for a moment. I talk to pemilik pabrik every day who are terrified of “compliance.” They buy a machine, start making bottles, and then panic when a big client asks for a certification they don’t have. It is even more stressful when you are importing equipment from China, like the machines I sell. You might wonder if the paperwork is real or if the standards match what you need in the US or Europe. I have spent years helping businesses set up compliant lines. I want to walk you through exactly what you need to know, so you can sleep at night knowing your bottles are safe.
What specific ISO and FDA certifications do I need for food-grade bottle production?
There is so much confusion about which piece of paper actually matters. Do you need a certificate for the machine? Or just the plastic? If you get this mixed up, you might spend money on the wrong things.
You need ISO 9001 for general quality consistency and ISO 22000 to manage food safety hazards. Crucially, the FDA does not certify machines; they regulate the resin and additives (21 CFR). You must ensure your raw materials meet these standards and your process follows Good Manufaktur Practices (GMP).

A stretch blow molding machine line produces PET beverage bottles under strict food grade and regulatory compliance.
The Difference Between Machine and Material
The first thing we need to clear up is a huge misconception. The FDA does not “approve” mesin cetak tiup. I cannot sell you a machine that is “FDA Approved” because that certification does not exist for machinery. The FDA approves the material that touches the food. This is called the “food hubungi substance.
Your responsibility is to ensure the plastic resin you buy—whether it is PET for water bottles or HDPE for milk jugs—complies with specific sections of the Code of Federal Regulations (CFR). For example, if you are using PET, it must meet the rules in 21 CFR 177.1630. If you are using HDPE, you have to look at 21 CFR 177.1520. These rules dictate exactly what chemicals can be in the plastic and how much is allowed to leak out.
ISO 9001 vs. ISO 22000: Building a System
While the FDA looks at the chemistry, ISO standards look at your management. You really need both to be a top-tier supplier.
ISO 9001 is about consistency. It proves that if you make a good bottle today, you can make the exact same bottle tomorrow. It forces you to document everything, from the temperature of the extruder to the pressure of the mold. If your machine drifts and the temperature gets too high, the plastic can degrade and become unsafe. ISO 9001 ensures you catch that drift before it becomes a problem.
ISO 22000 takes it a step further. This is specifically for food safety. It treats your bottle factory like a food factory. It requires you to have a plan for “prerequisite programs” like pest control, personal hygiene, and cleaning. It connects you to the food chain. If your resin supplier changes a formula, they have to tell you. If you change a setting that makes the bottle wall thinner, you have to tell the filler. It prevents the silence that leads to safety failures.
The “Food Contact Notification” (FCN)
Sometimes, you might want to add something new to your plastic, like a UV blocker to protect vitamin water. If this chemical isn’t already on the standard list, your supplier needs a “Food Contact Notification” or FCN. This is a specific approval from the FDA for that specific substance. You cannot just use a generic version. You need to trace every single additive in your masterbatch back to an FCN number. This is where many people fail. They buy cheap colorant that claims to be “food safe” but has no paperwork backing it up.
| Standar | Focus | Why You Need It |
|---|---|---|
| Kontak Pangan | Chemical Safety | Legal requirement for the resin itself. |
| ISO 9001 | Quality Management | Ensures you can repeat your results every time. |
| ISO 22000 | Food Safety Management | Integrates hygiene and hazard control into your business. |
| GMP | Bagus. Manufaktur Practices | Operational rules for cleanliness and safety. |
How do I verify if a Chinese blow molding machine manufacturer is truly ISO certified?
I know the market in China better than anyone, and I will be honest with you: fake certificates are a real problem. Getting a fake PDF via email is easy, but it offers you zero protection.
Never trust a PDF file alone. You must verify the certificate number on the official CNCA (Certification and Accreditation Administration of China) website. If it is not in that database, it is not valid. Also, look for the accreditation marks like UKAS or ANAB, and check for digital editing artifacts.
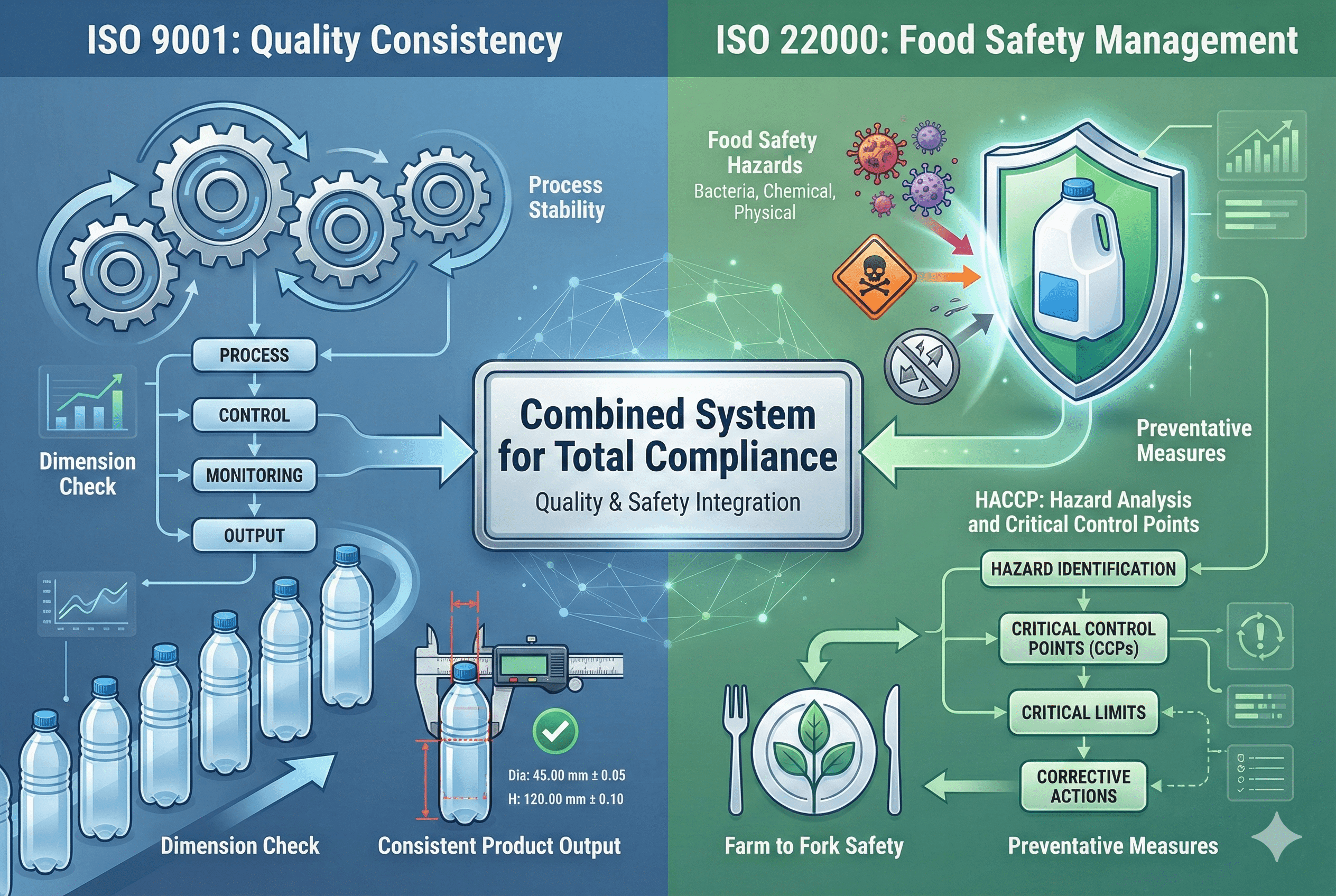
An infographic comparing ISO 9001 quality consistency and ISO 22000 food safety management in plastic bottle production.
The “Great Firewall” of Certification
In China, the government controls certifications very strictly through an agency called the CNCA. By law, every single valid ISO certificate issued to a Chinese company must be listed in the CNCA public database. This is your most powerful tool. When a supplier sends you a certificate, take the number and type it into that website.
If the database says “No Record Found,” the certificate is fake. It doesn’t matter what excuse the supplier gives you. Sometimes, you will find the record, but the “Scope” is different. This is a common trick called “Scope Creep.” A company might be certified for “Trading Plastic Goods” (meaning they are just a salesperson), but they photoshop the PDF to say “Manufacturing Plastic Goods.” The database will reveal the truth. If they aren’t certified to manufacture, they don’t have a quality system in place for building your machine.
Digital Forensics on Documents
You don’t need to be a detective to spot a bad fake, but you do need to look closely. Open the PDF and zoom in 400%.
- Look at the stamps: Real certificates are usually scanned physical papers. If the red stamp is perfectly vertical and computer-generated, while the signature looks like a vector graphic, be suspicious.
- Check the fonts: Does the company name look like it was typed in a slightly different font than the rest of the text? That suggests someone took a template and just swapped the name.
- Scan the QR Code: Many certificates have a QR code for verification. Scammers are smart; they will make a QR code that links to a fake website they built themselves. Always check the URL. If it doesn’t go to the official Certification Body (like SGS, TUV, or CQC), do not trust it.
The Only Real Proof: The Audit
Paperwork is just the first step. If you really want to know if a factory is following ISO standards, you have to ask for the boring stuff. Ask to see their “Management Review Minutes” from last year. Ask to see the “Calibration Logs” for their calipers and temperature sensors.
A company with a fake certificate will rarely go to the trouble of faking three years of boring meeting notes. If they say, “Oh, that is confidential,” that is a red flag. A real ISO factory is proud of their documentation. When I work with clients, I encourage them to visit the factory or hire a third-party auditor. Seeing the process in person is the only way to be 100% sure.
Why is FDA compliance critical for my plastic packaging business in the US market?
You might think skipping a few expensive tests is a smart way to save budget, especially when starting out. But in the US market, one bad bottle can literally destroy your entire company overnight.
FDA compliance is not optional; it is a legal requirement under the FD&C Act. If your bottles leach chemicals into food, they are considered “adulterated.” This triggers strict liability, meaning you are responsible for damages even if you didn’t know about the defect. Compliance protects you from bankruptcy.
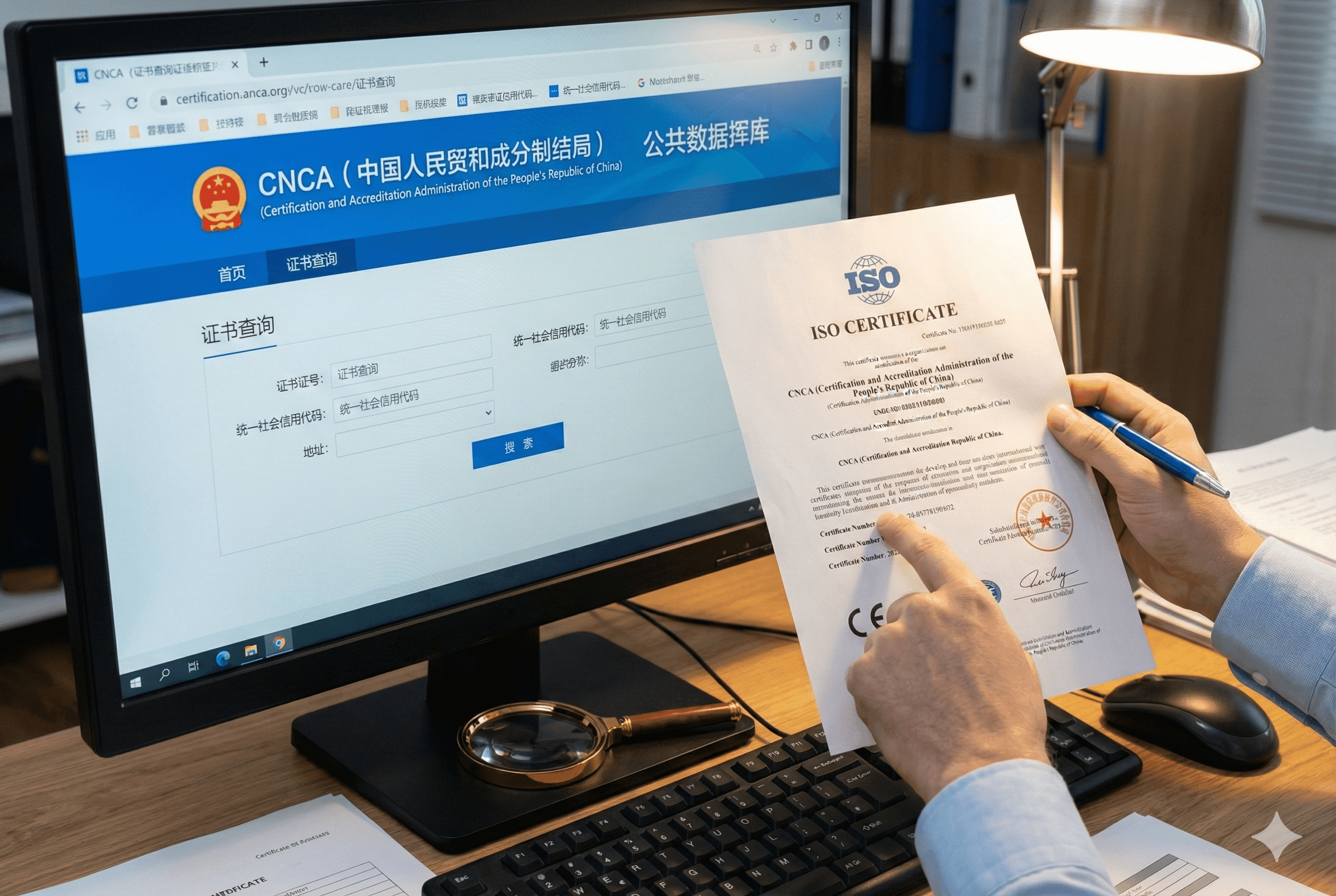
A quality manager checks an ISO certificate against the official CNCA government database to confirm compliance.
The “Adulteration” Trap and Strict Liability
The US law is extremely strict. Under the Federal Food, Drug, and Cosmetic Act, if a packaging material releases any harmful substance into the food, the food itself is considered “adulterated.” It is illegal to sell adulterated food.
Here is the scary part: the US operates on “strict liability.” This means a plaintiff (the person suing you) does not need to prove you were negligent or that you intended to hurt them. They only need to prove that your product was defective and it caused harm. You cannot say, “I didn’t know the machine was leaking oil.” That is not a defense. As the manufacturer or the importer of record, you absorb all the risk. If you are buying from overseas, the US courts often cannot reach the foreign factory, so they come after you.
Migration Testing: Proving Safety with Science
Compliance isn’t just a promise; it’s physics. We prove safety through “migration testing.” This involves putting the plastic in contact with chemicals that act like food, called “simulants.”
If you are bottling water, we use a simple alcohol solusi. But if you are bottling milk or cooking oil, it gets much harder. Fatty foods love to pull chemicals out of plastic. We use “Simulant D2” (usually vegetable oil) to test this. It is the “worst-case scenario.”
You also have to test for “Conditions of Use.” A bottle that is safe at room temperature might release toxins if you “hot fill” it with juice at 85°C. The heat opens up the molecular chains in the plastic and lets chemicals escape. If you only test at room temperature but sell the bottle for hot tea, you are breaking the law. You need to verify the “Specific Migration Limit” (SML) for dangerous things like Antimony or heavy metals. These limits are measured in parts per billion—tiny amounts that can cause big problems.
Brand Damage vs. Regulatory Fines
While the FDA can fine you, the real killer is the market. If a major brand buys your bottles and then has to recall their product because your plastic smelled funny or leached a chemical, they will sue you for the value of their lost product and the damage to their brand. That is usually in the millions of dollars. Your insurance might not cover it if you cannot prove you did your due diligence. Having a complete file of migration test reports is your only shield.
How do I ensure my production process maintains strict food-grade compliance standards?
Having the right papers in a drawer is one thing. Keeping the factory floor clean enough to eat off of is a totally different challenge, and it requires a change in mindset.
Operational compliance requires a Hazard Analysis Critical Control Point (HACCP) plan. You must control physical zones, air quality (ISO Class 8 cleanrooms), and use only H1 food-grade lubricants on your machines. Regular maintenance checks and “foreign material exclusion” protocols are vital to prevent physical contamination.

A laboratory technician prepares plastic bottle samples for migration testing to verify food contact safety.
Designing the Cleanroom Environment
Ketika Anda blow a bottle, you are injecting it with compressed air. That air touches the inside surface of the bottle—the surface that touches the food. If your factory air is full of dust, oil mist, or mold spores, you are injecting that directly into the consumer’s drink.
This is why we talk about ISO 14644 standards for air cleanliness. For most food packaging, you want an ISO Class 8 environment. This means you have strict filtration to remove dust. You also need to control “zoning.” You cannot open bags of raw dusty resin in the same room where the finished open bottles are cooling. You need physical walls and airlocks to keep the dirty zones separate from the clean zones.
Compressed air is a critical control point. You need filters that remove water (which grows bacteria), oil (from the compressor), and particles down to 0.01 microns. If you don’t treat your air like an ingredient, you are failing hygiene standards.
The Hidden Danger of Lubricants
Mesin blow molding are full of moving metal parts that need grease. Toggles, hydraulics, bearings—they all need lubrication. But what happens if a hydraulic hose bursts? Or a toggle drips grease?
In a food-grade facility, you must use the H1, H2, H3 classification system.
- H1 Lubricants: These are safe for “incidental food contact.” They are tasteless, odorless, and non-toxic. Every moving part above your mold or conveyor belt harus use H1 grease. If it drips, it’s not a disaster.
- H2 Lubricants: These are standard industrial greases containing toxic heavy metals. They perform better but are poisonous. You should ban these from your production floor if possible, so a mechanic doesn’t accidentally put toxic grease in the wrong gun.
Recycled Material (rPET) and the LNO
Everyone wants to be sustainable today, so you might be looking at using Recycled PET (rPET). This is great, but it introduces a huge risk. What if the plastic you are recycling came from a bottle that someone used to store pesticide or gasoline?
Standard washing doesn’t get those chemicals out. You need a “super-cleaning” process. The FDA issues something called a “No Objection Letter” (LNO) to recycling processes that prove they can clean up these contaminants. You cannot just buy cheap recycled flake from a broker. You must buy from a supplier who holds a valid LNO. If you use generic rPET for food bottles, you are creating a chemical hazard. You also need to verify that the LNO covers your specific use case. Some are only approved for room temperature, not hot fill.
Foreign Material Exclusion
Finally, you need to stop physical things from falling into bottles. This sounds simple, but it is a common failure. We call it “Foreign Material Exclusion.”
Do not allow employees to wear jewelry or watches. If a button falls off a shirt into a preform, it gets molded into the bottle. Use “metal-detectable” pens so if one drops, your metal detector kicks it out. And never use brittle acrylic plastic for machine guards. If a guard shatters, clear sharp plastic shards look exactly like botol plastic. Use polycarbonate instead—it dents, it doesn’t shatter. These small details are what separate a professional food-grade factory from a risky one.
Kesimpulan
Producing food-grade botol plastik is about the intersection of rigorous chemistry, strict law, and precise engineering. You cannot just trust a certificate; you must verify it through databases and audits. By understanding the FDA codes and implementing true hygiene standards like HACCP, you protect your business and your customers.
Peran Saya
I am Slany Cheuang, the Technical Sales Manager at Mesin LEKA. I specialize in helping manufacturers set up high-performance Extrusion and Stretch Blow Molding lines. I don’t just sell iron; I help you build a compliant, efficient production process that meets international standards. Whether you need a machine for complex jerry cans or high-speed water bottles, I guide you through the technical and regulatory landscape.
Target Audiens Saya
I write for factory owners, procurement managers, and production directors in the packaging industry. My readers are the people responsible for keeping the line running and keeping the product legal. You are likely based in North America, Europe, or emerging markets, looking to balance cost-efficiency with uncompromising safety standards.







0 Komentar